CHEMICAL LAWS OF COMBINATIONS
There are four laws of chemical combination which describe the general features of a chemical change.
(a) Law of conservation of mass: This law was established by Lavoisier, a French chemist. The law of conservation of mass states that matter is neither created nor destroyed during chemical reaction, but changes from one form to another.
Experiment to verify the law of conservation of matter (mass)
Theory:
The equation of the chemical reaction chosen for study is as follows:
Silver nitrate + sodium chloride → Silver chloride + Sodium trioxonitrate(v) (White precipitate)
Method:
1. Put some sodium chloride solution in a conical flask
2. Fill a small test tube with silver trioxonitrate (iv) solution of string, suspend it in a conical flask as shown below:
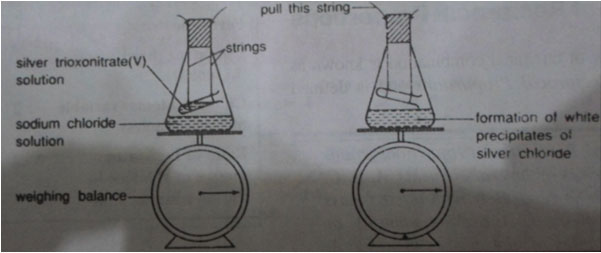 3. Insert the stopper and weight the whole apparatus on a balance, note the mass of the whole system.
3. Insert the stopper and weight the whole apparatus on a balance, note the mass of the whole system.
4. Mix the two liquids by pulling the string attached to the bottom end of the small test tube.
5.
- NEW: Download the entire term's content in MS Word document format (1-year plan only)
- The complete lesson note and evaluation questions for this topic
- The complete lessons for the subject and class (First Term, Second Term & Third Term)
- Media-rich, interactive and gamified content
- End-of-lesson objective questions with detailed explanations to force mastery of content
- Simulated termly preparatory examination questions
- Discussion boards on all lessons and subjects
- Guaranteed learning