TYPES OF ENERGY – KINETIC ENERGY
CONTENT
- The Kinetic Theory
- Diffusion
- The Basic Statements of the Kinetic Theory of Gases
The Kinetic Theory
The kinetic theory of matter assumes that matter is made up of tiny particles such as atoms, molecules and ions that are continually moving and therefore possess kinetic energy.
The kinetic theory combines the concept of heat as a form of energy with the molecular theory. First, matter is considered to consist of elastic particles that are in constant motion. By their motion, they possess kinetic energy. These elastic particles have become better known as molecules; and instead of the particulate theory of matter, we talk of the molecular theory of matter. Evidence of molecular motion is provided by diffusion and Brownian movement.
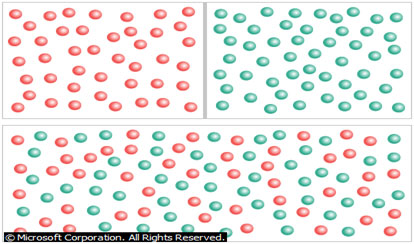
Diffusion
Diffusion is the natural tendency of molecules to flow from higher concentrations to lower concentrations. When the barrier between two substances is removed (as shown here), the molecules will diffuse throughout the entire container. While the number of molecules in the container is the same as it was before the barrier was removed, the substances are now at lower concentrations.
- NEW: Download the entire term's content in MS Word document format (1-year plan only)
- The complete lesson note and evaluation questions for this topic
- The complete lessons for the subject and class (First Term, Second Term & Third Term)
- Media-rich, interactive and gamified content
- End-of-lesson objective questions with detailed explanations to force mastery of content
- Simulated termly preparatory examination questions
- Discussion boards on all lessons and subjects
- Guaranteed learning