SOAPS AND DETERGENTS
CONTENT
- Soaps: Laboratory Preparation of Soap
- Structure of Soap
- Action of Soap as an Emulsifying Agent
- Cleaning Action of Soap
- Detergents: Preparation of Detergents, Mode of Action of Soap
- Differences between Soaps and Detergents
Soaps
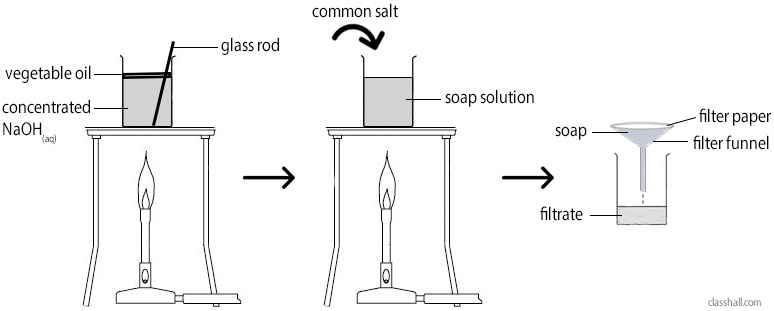
Laboratory Preparation of Soap
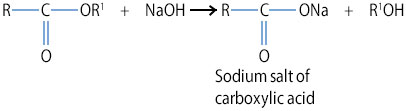
Soaps are saponification products of fats and oils. They are sodium salts of fatty acids. They are usually referred to as soapy detergents.
- A mixture of about 100cm3 of vegetable oil (palm kernel oil) and 100cm3 of 10% sodium hydroxide solution is boiled and agitated by passing steam until it is homogeneous.
- Saturated solution of sodium chloride (brine) is then added to the slightly cooked mixture, in order to salt out or separate the soap and from the liquid propan-1,2,3-triol (glycerol).
- The soap cord obtained is then slightly dried and mixed thoroughly with perfumes and covalent.
- Again, the soap is slightly dried, then pressed before being cut into tablets or bar.
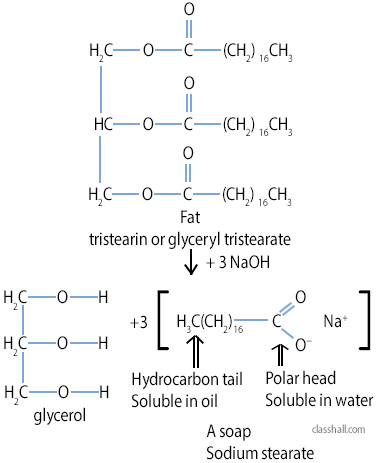
Structure of Soap
EVALUATION
- State the reason why sodium chloride is used during the manufacture of soap.
You are viewing an excerpt of this lesson. Subscribing to the subject will give you access to the following:
- NEW: Download the entire term's content in MS Word document format (1-year plan only)
- The complete lesson note and evaluation questions for this topic
- The complete lessons for the subject and class (First Term, Second Term & Third Term)
- Media-rich, interactive and gamified content
- End-of-lesson objective questions with detailed explanations to force mastery of content
- Simulated termly preparatory examination questions
- Discussion boards on all lessons and subjects
- Guaranteed learning