THE PERIODIC TABLE OF ELEMENTS
CONTENT
- The Periodic Table
- The Periodic Law
- Electronic Configuration of the First Thirty Elements
- Meaning of Atomic Orbital
- Rules and Principles for Filling in Electrons
- Blocks of Elements
- Characteristic Properties of Transition Elements
- Families of Elements
- Periodic Properties
The Periodic Table
This shows the arrangement or grouping of elements in order of increasing atomic number.
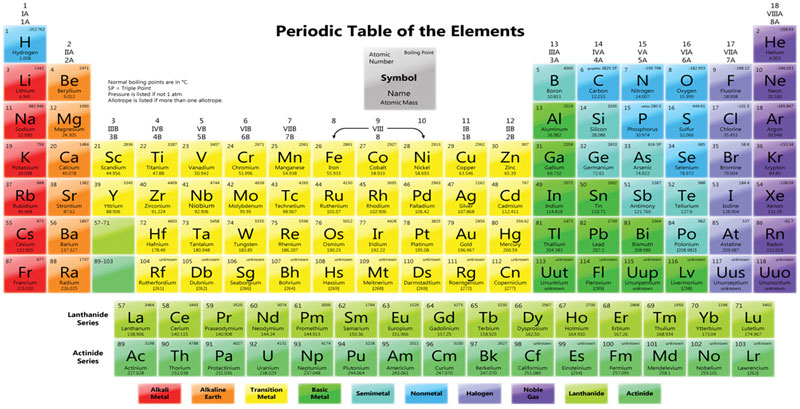
The Periodic Law
This is the basic assumption behind the modern periodic table; it states that the properties of the elements are the periodic function of their atomic number.
Electronic Configuration of the First Thirty Elements
The electronic configuration of an atom is the representation of the arrangement of the electrons distributed among the orbital shells and subshells. Commonly, the electronic configuration is used to describe the orbitals of an atom in its ground state.
Meaning of Atomic Orbital
Orbital is the region of space around the nucleus where there is a high probability of finding electron.
You are viewing an excerpt of this lesson. Subscribing to the subject will give you access to the following:
- NEW: Download the entire term's content in MS Word document format (1-year plan only)
- The complete lesson note and evaluation questions for this topic
- The complete lessons for the subject and class (First Term, Second Term & Third Term)
- Media-rich, interactive and gamified content
- End-of-lesson objective questions with detailed explanations to force mastery of content
- Simulated termly preparatory examination questions
- Discussion boards on all lessons and subjects
- Guaranteed learning