HEAT ENERGY: TRANSFER OF HEAT
CONTENT
Conduction of Heat
- Meaning of Heat Conduction
- Using Kinetic Molecular Theory to Explain Conduction in Solids
- Conduction of Heat in Liquids
- Experiment to Show that Water is a Poor Conductor of Heat
- Applications of Conductors and Insulators
Convection of Heat
- Meaning of Convection of Heat
- Using Kinetic Molecular Theory to Explain Convection in Liquids
- Applications of Convection
Radiation, Emission and Radiation by Different Surfaces
Conduction of Heat
Meaning of Heat Conduction
Conduction of heat is the process by which heat is passed along a material from molecule to molecule while the heated particles remaining in mean position. Most metals are good conductors but their thermal conductivities differ from one metal to another. Experiment performed to compare the conductivity of solid showed that copper is a better conductor than brass, followed by iron, lead…
Using Kinetic Molecular Theory to Explain Conduction in Solids
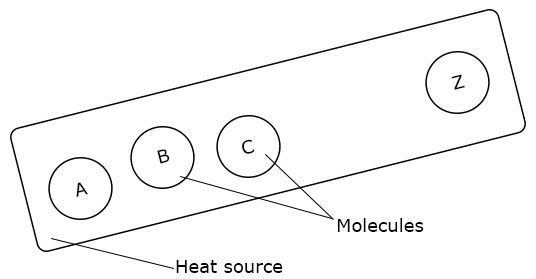
When the end A is heated, molecule A vibrates about its mean position with a greater kinetic energy and pushes the molecule B to do the same.
You are viewing an excerpt of this lesson. Subscribing to the subject will give you access to the following:
- NEW: Download the entire term's content in MS Word document format (1-year plan only)
- The complete lesson note and evaluation questions for this topic
- The complete lessons for the subject and class (First Term, Second Term & Third Term)
- Media-rich, interactive and gamified content
- End-of-lesson objective questions with detailed explanations to force mastery of content
- Simulated termly preparatory examination questions
- Discussion boards on all lessons and subjects
- Guaranteed learning